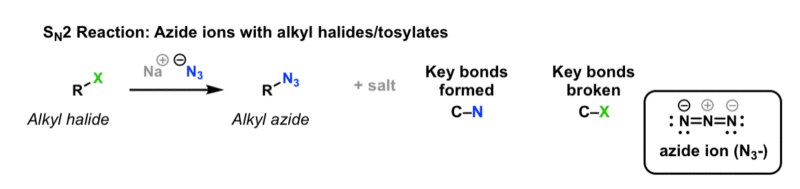
Sn2 Vinylic Halides

There are many cases where allylic halides react preferentially by an mathrm s n 1 process.
Sn2 vinylic halides. From the perspective of applications the dominant member of this class of compounds is vinyl chloride which is produced on the scale of millions of. S n 2 reactions of allylic halides and tosylates. A sn1 sn2 mechanism on vinyl halide would look like this. To understand why vinylic and aryl halides are inert under s.
Steric hindrance caused by the benzene ring of the aryl halide prevents s n 2 reactions. In addition the carbon halogen bond is shorter and therefore stronger in aryl halides than in alkyl halides. The student asked why do vinyl halides not do the sn2 reaction my answer was that two reasons exist for why the vinyl halide will not react with a nucleophile. Rapid s n 2 substitution for 1º halides note there are no β.
Allylic halides and tosylates are excellent electrophiles for bimolecular nucleophilic substitution reactions s n 2. The picture below helps explain why this reaction is so much more difficult energetically more costly than the more common solvolysis of an alkyl halide. In organic chemistry a vinyl halide is a compound with the formula ch 2 chx x halide the term vinyl is often used to describe any alkenyl group. Classification allyic vinylic benzylic aryl halides.
The substituents around a double bond are within the same plane therefore an s math n math 2 would give steric hindrance. Likewise phenyl cations are unstable thus making s n 1 reactions impossible. A s math n math 2 mechanism is not favoured for 3 reasons. Today i got a good question i want to make a point of posting the best question from the day s teaching and my answer.
We can shift from one mechanism to the. Chemistry concept 2 058 views. Certain vinylic halides can be forced to react by the s n1 e1 mech anism under extreme conditions but such reactions are relatively uncommon. The carbon halogen bond is shortened in aryl halides for two.
In high dielectric ionizing solvents such as water dimethyl sulfoxide acetonitrile s n 1 and e1 products may be observed. They exhibit faster s n 2 reactivity than secondary alkyl halides because the bimolecular transition state is stabilized by hyperconjugation between the orbital of the nucleophile and the conjugated pi bond of the allylic. Vinylic and aryl halides however are virtually inert to the conditions that promote s n1 or e1 reactions of alkyl halides. The resultant vinylic carbocations are actually stable enough to be observed using nmr spectroscopy.
Rapid s n 2 substitution for 1º and 2º halides. For this reason alkenyl halides with the formula rch chx are sometimes called vinyl halides. Nucleophilic substitution reactions sn1 and sn2 mechanism. Since both the allylic mathrm s n 1 and mathrm s n 2 reactions are stabilized there is a delicate balance between the two pathways.
Why do allylic halides prefer sn2 reaction over sn1.