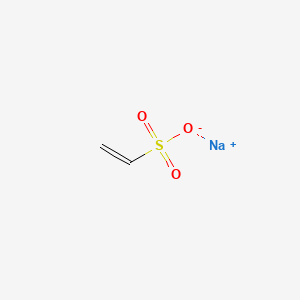
Sodium Vinyl Sulfonate Structure

Linear formula ch 2 chso 3 na.
Sodium vinyl sulfonate structure. Sulfonates are the conjugate base of sulfonic acids. Do not give sodium polystyrene sulfonate orally by mouth to a newborn baby. Beilstein reaxys number 3711195. Sulfonates are generally stable in water non oxidizing and colorless.
Sodium vinyl sulfonate 25 svs has both an olefinic bond and a reactive sulfonic acid group. Sodium vinyl sulfonate sodium vinyl sulfonate svs has both an olefinic bond and a reactive sulfonic acid group. Linear formula c 2 h 3 nao 3 s n. Sodium vinyl sulfonate polymer.
Pvsa cas number 9002 97 5. Vinyl sulfonate sodium salt. Sodium polystyrene sulfonate can be given as a liquid by mouth through a stomach feeding tube or as a rectal enema. This bifunctional structure suggests its applications ranging from its use as a monomer in the polymerisation of sulfonated vinyl type resins to its use as an organic intermediate in sulfoethylation reactions.
The study group consisted of 53 workers 39 female aged 18 to 51 working in two factories in the peoples republic of china that manufactured sodium allyl sulfonate. Ethylenesulfonic acid sodium salt sodium vinylsulfonate solution vinylsulfonic acid sodium salt cas number 3039 83 6. In h 2 o technical grade synonym. In h 2 o technical grade synonym.
Vinylsulfonic acid sodium salt solution 25 wt. Pubchem substance id 24856814. Molecular weight 130 10. A sulfonate is a salt or ester of a sulfonic acid.
Poly vinylsulfonic acid sodium salt solution 30 wt. Do not use the medicine orally or rectally in a baby who has slow digestion caused by surgery or by using other medicines. Many useful compounds and even some biochemicals feature sulfonates. Sodium vinyl sulfonate 25 svs is used as polymerizable non migratory surfactant or copolymer in emulsion polymerization.
An epidemiological survey of the effects of occupational exposure to allyl chloride was conducted. Sodium vinyl sulfonate is a polymerizable organic compound which has many industrial uses particularly as an antistatic agent and as an agent improving the tinctorial affinity of synthetic fibres such as polyacrylonitrile and polypropylene to cationic dyes as a dispersing agent in polymer emulsions. It contains the functional group r so 3 where r is an organic group. This bi functional structure suggest its applications ranging from its use as a monomer in polymerization of.